Food Packaging regulations in Europe
Find out more about the differences between the European Union, Germany, Switzerland, Turkey, Russia, Ukraine and Israel regarding the legislation on food packaging materials. For each country we have summarized several details on its individual regulations.
European Union
Thanks to a harmonized legislation on food packaging materials in the EU, it’s equally applicable in each of currently 28 member states. Learn more about different types of regulations concerning the protection of human health and consumer interests, associated processes and responsibilities.
European Union
In the European Union, currently comprising 28 member states, legislation on food packaging materials is harmonized and thus equally applicable in each member state. The Framework Regulation (EC) No 1935/20041 related to materials and articles intended to come into contact with foodstuffs provides the basis for the assurance of a high level of protection of human health and of consumers’ interests in relation to food packaging, whether printed or not. The manufacturer of the final packaging is responsible for the compliance of the material and the article with the legal requirements laid down in Article 3:
Materials and articles […] shall be manufactured in compliance with Good Manufacturing Practice so that, under normal or foreseeable conditions of use, they do not transfer their constituents to food in quantities which could:
- endanger human health; or
- bring about an unacceptable change in the composition of the food; or
- bring about a deterioration in the organoleptic characteristics thereof.
1 REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC, OJEU L338 of 13.11.2004
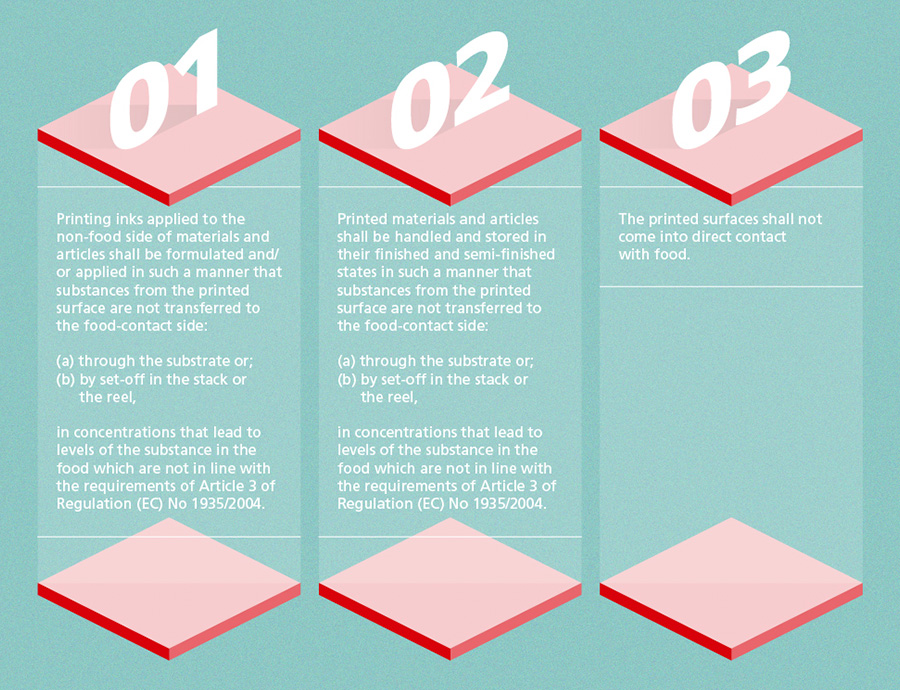
The GMP Regulation (EC) No 2023/20062 lays down rules on Good Manufacturing Practice for materials and articles intended to come into contact with food. It introduces general rules for all business operators in the supply chain, and specifies that quality assurance and control systems are established and implemented. All printing inks intended for use on food packaging are within the scope of this regulation. The Annex introduces detailed rules which relate to processes involving the application of printing inks to the non-food side of a material or article3:
2 Commission Regulation (EC) No 2023/2006 of 22 December 2006 on Good Manufacturing Practice for materials and articles intended to come into contact with food, OJEU L384 29.12.2006
3 For more information, see the “EuPIA Position on Regulation (EC) No 2023/2006 of 22 December 2006 on Good Manufacturing Practice for materials and articles intended to come into contact with food”, www.eupia.org
There is today no specific EU legislation concerning printing inks for food packaging. The European Commission informed in December 2016 that they intend to adopt a new Union legislation on printed Food Contact Materials (p-FCM). Currently, an extensive stakeholder dialogue is taking place which is due to the complexity of the FCM supply chain quite timeconsuming. Furthermore, the envisaged p-FCM regulation will only be the second regulatory initiative on harmonizing FCMs on European level. Consequently, all stakeholders of not yet harmonized FCMs (e.g. adhesives, paper and board, etc.) do see the p-FCM as pilot legislation serving as blueprint for their respective segments later on. For that reasons an adoption of the regulation will not take place in 2018 as originally planned.
Therefore the main specific regulation pursuant to the Framework Regulation is still the Regulation (EU) No 10/20114 with its subsequent amendments on plastic materials and articles intended to come into contact with foodstuffs. It lays down an overall migration limit (OML) of 60 mg/kg food. In addition, specific migration limits (SMLs) or maximum content in the material or article (QM) are set for individual substances.
The regulation contains a positive list of monomers and other starting substances as well as of additives. Substances used only in the manufacture of printing inks are not listed, and thus packaging inks are not under the scope of this regulation. However, ink on printed plastic packaging is covered if it contains components which are listed (thus are socalled evaluated substances), therefore the relevant restrictions, such as specific migration limits (SMLs) or maximum content (QM) have to be met by the final packaging (which includes the possible effect of certain ink substances). Finally, Article 8 provides for substances used for plastic layers in plastic food 'contact materials that they shall be of a suitable technical quality and purity. This requirement does not directly cover substances used for inks, however, it appears advisable to observe it in the light of Article 3 of the mentioned Framework Regulation (EC) No 1935/2004, which includes printed layers as part of the final packaging.
4 Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food, OJ L 12 of 15.01.2011
Council of Europe (CoE)
The CoE covers, with currently 47 countries, an area which is bigger than the EU (www.coe.int). For decades, in an attempt to harmonize regulations in Europe, expert committees representing officials from the member countries have been working on food contact materials to issue proposals for regulations which are mostly based on positive lists. However, the CoE itself cannot issue laws; furthermore, it is a fact that member countries have only partly used these models (called ‘Resolutions’) for writing their own regulations and guidances for industry in the field of food contact materials. In the field of colorants, the CoE issued, in 1989, a document which was widely recognized as a workable standard. Resolution AP(89)1 “on the use of colorants in plastic materials coming into contact with food” was established with the support of the European pigment and dye manufacturers; consequently, it became an integrated part of industry standards and processes. Its specifications for limits for impurities in the colorants are regarded to be the benchmark for ink pigments as well despite the fact that its intended scope is restricted to mass coloration of plastics which are, in contrast to printed layers, in direct contact with food.
The CoE also published, in 2005, Resolution AP(2005)2 on "Packaging Inks Applied to the Non-Food Contact Surface of Food Packaging". It includes a positive list of substances allowed for use in inks. However, since it is largely incomplete and does not reflect current practices, it is not usable, either by regulators nor by industry.
Introduction/Legal compliance
On 7th December 2021, the so-called German "Printing Ink Ordinance" was published in the Federal Bulletin and came into force on 1st January 2022. However, this does not mean, that the provisions of this ordinance will automatically apply from this date. A general transition period of four years until 1st January 2026 was granted until the legal provisions will apply on the market.
This implies, that for the time being inks and lacquers for food contact materials remain legally compliant under the current provisions and that compliance declarations (e.g. Statement of Composition – SoC) remain valid until further notice. Consequently, this also applies to all compliance declarations further down the Food Packaging Supply Chain.
Scope
Although the term "Printing Ink Ordinance" has become popular, the Ordinance does not only regulate printing inks as such, but printed “Food Contact Materials” (FCM) for which a transfer of substances from the printing ink layer to the food cannot be excluded. The primary addressee of the ordinance is therefore the commercial manufacturer of printed FCM. The scope includes applications where the printing ink layer is in direct contact with the foodstuff as well as those where the printing ink layer is applied on the non-food contact side of the material.
Details
The core of the German ordinance is an ink component positive list: Table 1 lists 540 substances which may be used for the manufacture of printing inks for FCM; Table 2 lists 54 additional pigments for transient „Direct Food Contact“ (DFC) – which remains valid for a transition period of five years before becoming obsolete. In the positive list, specific migration limits (SML), group limits or restrictions are partially laid down, which must be observed. If no migration limit or other restrictions are defined, the global migration limit of 60 milligrams per kilogram of the foodstuff applies.
Non-listed substances (NLS) may be used as long as a) the printing ink layer is not in direct contact with a foodstuff, b) the substances used are not classified as CMR under chemical legislation (CLP Regulation) and c) the migration level is below 10 ppb. Furthermore, non-intentionally added substances (NIAS) must be evaluated in accordance with internationally recognised scientific principles of risk assessment, a provision already required according to the EU Framework Regulation and long incorporated into Siegwerk SoCs.
Importance of the transition period
It has to be pointed out that the present “Printing Ink Ordinance” currently lacks proper substance evaluations and that the positive list is incomplete, as it does not include some essential substances. These deficiencies have been acknowledged by the legislator in allowing for the 4 year transition period. Time will be needed in order to clarify these issues, which affect the entire value chain, be that in regards to the procedures for notification of adding substances to the positive list, or linked to conformity aspects of individual print and packaging designs, where a variety of options for compliant solutions are available.
Switzerland was the first country to issue a specific ink legislation in 2008, the so-called “Swiss Ordinance”. This ordinance introduced for the first time a positive list for food packaging inks. In the meantime the Swiss Federal Department of Home Affairs (FDHA) issued a revised version of the “Ordinance on Materials and Articles intended to come into contact with foodstuffs” (quoted as SR 817.023.21)1, which came into force on 1st May 2017 with a transition time of 4 years. Section 12 sets out the provisions relating to printing inks applied on the non-food contact surface of Food Contact Materials and article 35 of this section details the requirement that only permitted substances should be used in the manufacture of these inks.
Permitted Substances are defined as those which are listed in Annex 2 and in Annex 10. The positive list of Annex 10 contains in the actual valid version (V 1.1) 5290 entries with a corresponding classification of the substances in Part A (toxicologically evaluated) or Part B (non-evaluated).
Part A substances are listed with Specific Migration Limits (SMLs) or without a specific limit, in which case the OML of 60 ppm applies. These substances may be used in the manufacture of inks, given that the SMLs are not exceeded for the final packaging.
Part B substances are only permitted to use if they are not classified as "CMR" substances (carcinogenic, mutagenic, reprotoxic = classes 1A, 1B or 2 acc. to the CLP Regulation) and if their migration is not detectable with a detection limit of 10 ppb.
It has to be pointed out, that the Swiss Ordinance is applicable only for Intentionally Added Substances (IAS) and that it does not cover direct food contact (DFC) inks. Siegwerk has ensured and continues to ensure, in Europe, that all raw materials which are used in printing inks and varnishes intended for food packaging are included in this positive list. Despite the fact that the regulatory obligation is only legally binding for Switzerland and relevant for the packaging market in Europe, Siegwerk has safeguarded that the large majority of the raw materials used worldwide are listed as well.
1 Ordinance of the FDHA on materials and articles intended to come into contact with foodstuffs (SR 817.023.21), Eidgenössisches Departement des Innern (EDI), 16.12.2016, www.admin.ch
Turkey
Turkey has implemented the relevant EU regulations on food contact materials in national regulations (www.tarim.gov.tr). The "Turkish Food Codex Regulation on Materials and Articles" (No. 30382 of 05.04.2018) covers similar principles as the "Framework Regulation" 1935/2004, whereas the "Registration procedure for food contact materials and substances manufacturing enterprises and Good Manufacturing Practice" (R.G. 03.08.2012) consist of "GMP Regulation" 2023/2006 exactly.
The "Regulations of food contact plastics materials and substances" (Teblig No: 2013/34) and the "Regulation of list of food simulants used for food contact plastics materials and substances" (Teblig No: 2013/35) are similar to the "Plastics Regulation" 10/2011 in the EU. Therefore, in Turkey the same migration control principles, positive lists and thresholds are in place.
Russia
In 2013 the Russian Federation issued a draft Technical Regulation on the Safety of Materials in Contact with Food. In this regulation, for example, there is no general positive list approach. However, for many polymers, Permissible Quantities of Migration (PQMs) have been established. These lists are not exhaustive, as there are no PQMs for starting monomers or for most additives. A declaration of conformity is obligatory for all materials in contact with food products. The migration of harmful substances emitted by materials in contact with food products made of composite materials is investigated only for the layer directly in contact with food products. Regarding inks, it can be concluded that those not in direct contact with food are not included in the scope of the regulation today. The Hygienic Normative 2.3.3.972-00 specifies maximum permissible quantities of chemical substances allowed to migrate from materials in contact with foodstuffs.
Ukraine
Important regulations are the following:
Law of Ukraine “On Amendment of Certain Legislative Acts of Ukraine Regarding Food Products” No. 1602-VII of 22 July 2014
Sanitary Norms and Regulation 42-123-4240-86 “Migration Limits for Chemicals Released from Polymeric and Other Materials that Come into Contact with Food and methods of Determining Them” (12/1986)
Israel
Israel regulates food-contact materials based on EU and FDA standards, according to Israel Standard SI 5113 (“Plastic Materials and Plastic Articles in Contact with Food and Beverages”) (2002), Section B, paragraph 2.1. Where a particular material or article is not regulated by the EU or FDA, SI 5113 provides that it must comply with the requirements of the Israel Ministry of Health

