With consumer safety as key priority, we have established a comprehensive raw material introduction process. It is based on a centrally coordinated approval via our global PSR team and operated on a worldwide basis:
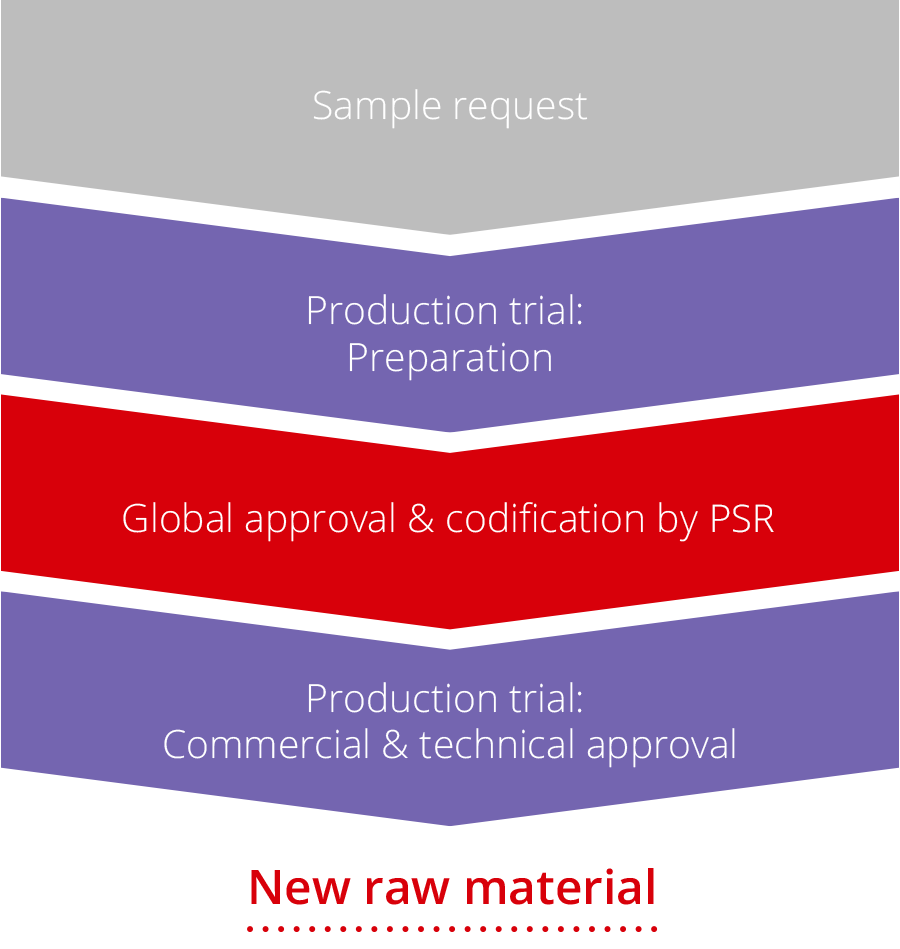
With this qualification process we strive to achieve full knowledge about the chemical composition of all raw materials intended for nutrition, pharma and hygiene (NPH) applications. Furthermore, the process shall ensure an efficient introduction of new raw materials or existing materials for new applications.
Supplier qualification
Our suppliers must first of all be qualified. During our global approval process, which is based on data received via the Siegwerk Raw Material Approval Form, we request and assess information on:
- Compliance with exclusion criteria (e.g. carcinogenics, mutagenics, repro-toxics, toxics)
- Compliance with our stringent purity standards (e.g. heavy metals, dioxins, primary aromatic amines)
- Compliance with chemical registration in each applicable region
- Composition data for NPH applications
These standards go far beyond legal requirements and are success factors for highest possible product safety.
Formulation guideline
The approval criteria for raw materials that are used in the production of printing inks for food packaging are particularly stringent. Top priority here is compliance with the relevant provisions on consumer protection with respect to the packaged food. To achieve the best possible control over the use of raw materials in the variety of possible formulations, we have developed a detailed internal formulation guideline that encompasses our extensive knowledge on raw materials.
Using a traffic light system, we provide recommendations on raw materials to colleagues around the world that are ultimately responsible for developing our printing inks. This ensures that our packaging inks only include raw materials that have been thoroughly checked beforehand.
Global Siegwerk code
Once the particular raw material has passed the qualification process successfully, we assign a unique Siegwerk code to it. This is the official authorization for purchasing and afterwards using the raw material in the formulation of our printing inks. If there are any doubts, however, we reserve the right to reject raw materials or perform our own analytical testing. Following clear, internally defined rules, supplier audits are a further tool for validation of the raw material portfolio. All these processes guarantee the safety of our printing inks and enable the customer to manufacture a finished product that meets the legal requirements.

