NIAS (Non-intentionally added substances)
The terms Nias resp. NIAS can be found frequently in our daily life. For example, Nias is an Indonesian island located in the Indian Ocean. NIAS, on the other hand, is also an abbreviation for e.g. the Northern Ireland Ambulance Service, the Nordic Institute of Asian Studies, the Netherlands Institute for Advanced Study in the Humanities and Social Sciences or the Northern Inland Academy of Sport.
When we are speaking of NIAS at Siegwerk, we always refer to so-called non-intentionally added substances. On this page you will learn more about NIAS in the context of food contact materials and printing inks, including definitions, different categories as well as the handling of NIAS at Siegwerk.
Scope and definitions
Non-intentionally added substances are chemical compounds that, in contrast to IAS (intentionally added substances), are present in a material but which have not been added intentionally within the production process. They can be by-products or impurities of starting substances, for example. Learn more about these and related topics by reading our ePaper.
Non-intentionally added substances are chemical compounds that, in contrast to IAS (intentionally added substances), are present in a material but which have not been added intentionally within the production process. NIAS can be by-products or impurities of starting substances, for example. The following graphic provides a general overview on IAS and NIAS:
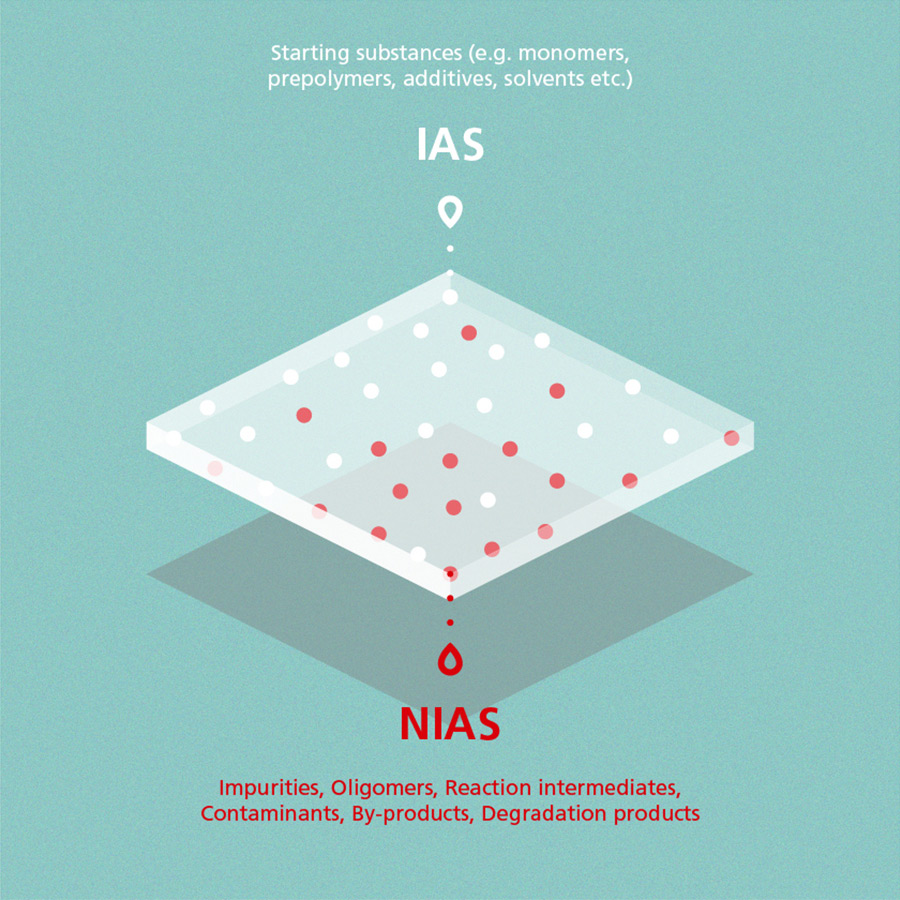
Definitions
According to the “Union Guidelines on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food”, NIAS are defined as follows:
“[…] an impurity in the substances used or a reaction intermediate formed during the production process or a decomposition or reaction product.”
The ILSI “Guidance on Best Practices on the Risk Assessment of Non-intentionally Added Substances in Food Contact Materials and Articles” even broadens the scope for NIAS to include contaminants from production processes or from the environment.
The perspective changes what you see
Depending on the position of a manufacturer in the packaging chain, the perception on what is a NIAS and what is not may vary. For example, a monomer is an IAS from the point of view of a raw material producer as it is intentionally used to form the polymer. However, from a printing manufacturer’s point of view – who uses, for example, this polymer dispersion as ink ingredient – the residual monomers are not intentionally added to the ink; they are just an unavoidable part of the raw material that was used, thus NIAS.
There is not always an exact border in determining IAS and NIAS. As a conclusion, it can be stated that the perspective changes what you see. Although the substance is the same, it may be perceived as an IAS or a NIAS depending on the manufacturer’s perspective within the packaging chain. This is well reflected in the following chart:
Risk assessment and categories of NIAS
The Union Guidelines on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food” also states:
“In principle, non-intentionally added substances will have to comply with the general safety requirements of Article 3 of the Framework Regulation and are subject to risk assessment in line with Article 19 of the Plastics Regulation.”
Basic principles of Article 3 of the Framework Regulation 1935/2004 are that there shall be:
- no risks related to human health
- no changes in the composition of the food
- no deterioration in the organoleptic characteristics thereof
Of course, these principles apply to IAS and NIAS in the same way. In the end, what matters is that the final Food Contact Material fulfils Article 3 of the Framework Regulation. This is irrespective of a substance being IAS or NIAS. As a consequence, NIAS need to be subject to a risk assessment to ensure that the consumer is not harmed by any substances migrating from the packaging material into the food. There are different possible scenarios regarding such risk assessments, which can be subdivided into the following categories:
- Identified NIAS with known chemical structure; toxicity data available
- Identified NIAS with known chemical structure; toxicity data not or not fully available
- Detected NIAS with unknown chemical structure
- Unknown NIAS
While for categories 1 and 2 a risk assessment is possible, for category 3 this is not practicable. However, screening via bioassays might be applied here. For category 4, a risk assessment is not possible at all.
Handling of IAS and NIAS at Siegwerk
How does Siegwerk deal with IAS and NIAS? Within our legal compliance check, for all IAS the respective migration limits apply. For NIAS, there are three different scenarios in accordance with the Swiss Ordinance on materials and articles in contact with food. Scenario 1 is that there is an SML based on an evaluation (Annex 6, Part A). In this case, Siegwerk uses the particular SML and no risk assessment is required. Scenario 2 is that there is a substance that is unevaluated (Annex 6, Part B). In this case, Siegwerk’s Food Safety Team performs a risk assessment to obtain a self-derived SML for that substance. Finally, scenario 3 is that the substance is neither listed in Part A nor in Part B. In this case, Siegwerk also performs a risk assessment.
Our responsibility for NIAS already starts at raw material level. We strive to achieve complete knowledge of the chemical composition of all raw materials based on the intended packaging applications down to trace level. Thus, we explicitly request information on NIAS from our raw material suppliers via our global raw material approval forms. On the other hand, we provide information on potentially migrating substances to our customers via our Statements of Composition (SoC). Siegwerk can therefore be seen as both a recipient as well as a provider of information. This flow of information is illustrated within the following chart:
NIAS – Flow of information along the supply chain





